Tumor Immunotherapy Program
About TIP
The Tumor Immunotherapy Program (TIP) at the Princess Margaret Cancer Centre is the first program of its kind in Canada and one of a handful around the world. Our team is made up of experts in the fields of immunology, oncology, genomics, bioinformatics, pathology and surgery.
TIP is directed by Dr. Pamela Ohashi, a world-renowned basic and translational immunologist and Dr. Lillian Siu who integrates TIP with two other key programs at the Princess Margaret: the Drug Development Program (DDP) and Cancer Genomics Program (CGP). These three programs form a synergistic triad that supports the Princess Margaret Cancer Centre’s core vision to deliver precision cancer medicine.
The four key research domains of TIP include:
- Discovery research.
- Investigator-initiated clinical studies.
- Sponsored clinical studies.
- Health services and outcomes research.
Our Mission
The mission of the Princess Margaret Tumor Immunotherapy Program is to harness the immune system to help conquer cancer.
Achieving our Mission
In order to achieve our mission, we perform research that is aimed at:
- Discovering how to engage the immune system to fight cancer.
- Profiling the immune system of patients with cancer.
- Developing new therapies in the laboratory.
- Testing new therapies in the clinic.
- Improving the quality of life for patients with cancer.
In the News
New clinical trial:
We have recently received approval for the launch of a new immune therapy trial, anticipated to open at the end of March 2016.
Our TIP researchers and physicians have developed this flagship trial in partnership with Merck & Co., Inc. Led by Dr. Lillian Siu and Dr. Pamela Ohashi, this trial will accrue 100 patients with different types of advanced solid tumors. Patients will be treated with a new immune therapy drug called pembrolizumab, which targets a protein in the immune system called PD-1. This drug is a type of immune checkpoint blockade, that ‘releases the brakes’ on the immune system, thereby enhancing the cancer-fighting activity of the immune system.
One major aspect of this trial will be the in-depth immune monitoring of all patients. Tumor and blood samples will be analyzed with cutting-edge methods. Valuable information will be gained about how this immune therapy drug impacts the immune profile of different patients and the immune profiles specifically associated with successful therapy. This study also includes sophisticated genomic profiling, in order to understand the genetics of cancer cells and immune cells and how specific genetic features are related to treatment outcomes.
The study will be open to patients with the following tumor types:
- Squamous Cell Cancer of Head and Neck (SCCHN)
- Triple Negative Breast Cancer (TNBC)
- Epithelial Ovarian Cancer (EOC) Type II
- Malignant Melanoma (MM)
- Advanced Solid Tumors
This clinical trial is registered at https://clinicaltrials.gov (NCT02644369)
For further information on this trial please use one of the following contacts:
- Email: TIP@uhn.ca
- Hotline for physician referrals: (416) 946-4501 x 5885
Featured in The Globe and Mail:
An article in The Globe and Mail entitled: “Scientists unleash the power of immunotherapy on stubborn cancers” described advances in cancer immunotherapy and some of the researchers in the field, including the Director of TIP, Dr. Pamela Ohashi.
Leading Features
Developing New Therapies:
TIL therapy: We are the first group in Canada to perform clinical trials of a new type of immune therapy called “TIL therapy” (TIL stands for Tumor-Infiltrating Lymphocyte). TILs are specialized immune cells (T cells) that are capable of fighting cancer cells. In TIL therapy, a patient’s own TILs are activated and multiplied in the laboratory. The TILs are then given back to the patient. The TILs that are used in our clinical trials are produced by our Cell Production Team. We have launched trials for patients with metastatic melanoma, ovarian cancer, and mesothelioma.
TCR gene transfer trial: We are planning to open the first clinical trial in Canada that uses genetically engineered T cells. These T cells are referred to as “TCR gene-transduced T cells”. These T cells will be manufactured in the laboratory so that they can potentially kill off cancer cells when given to patients.
A new type of cell: Drs. Marcus Butler and Naoto Hirano have produced a cell called an “artificial antigen-presenting cell” (aAPC). These aAPCs will be used in the laboratory to grow T cells that can kill cancer cells.
Developing the next generation of immune therapies: We are conducting studies to understand how a T cell response can be regulated and augmented in order to improve its ability to kill cancer cells. There are many types of cells and molecules that have the ability to either promote a T cell response, or inhibit it. Unraveling these complex interactions will identify novel regulators that can be targeted in the next generation of therapeutic agents.
Developing new clinical trials of combination therapies: While current immune therapies are generally aimed at improving one aspect of the immune response, combining different therapeutic strategies could further improve the immune response and result in a stronger and more sustained attack on a patient’s cancer. Our team is working on multiple types of combination approaches to treat cancer.
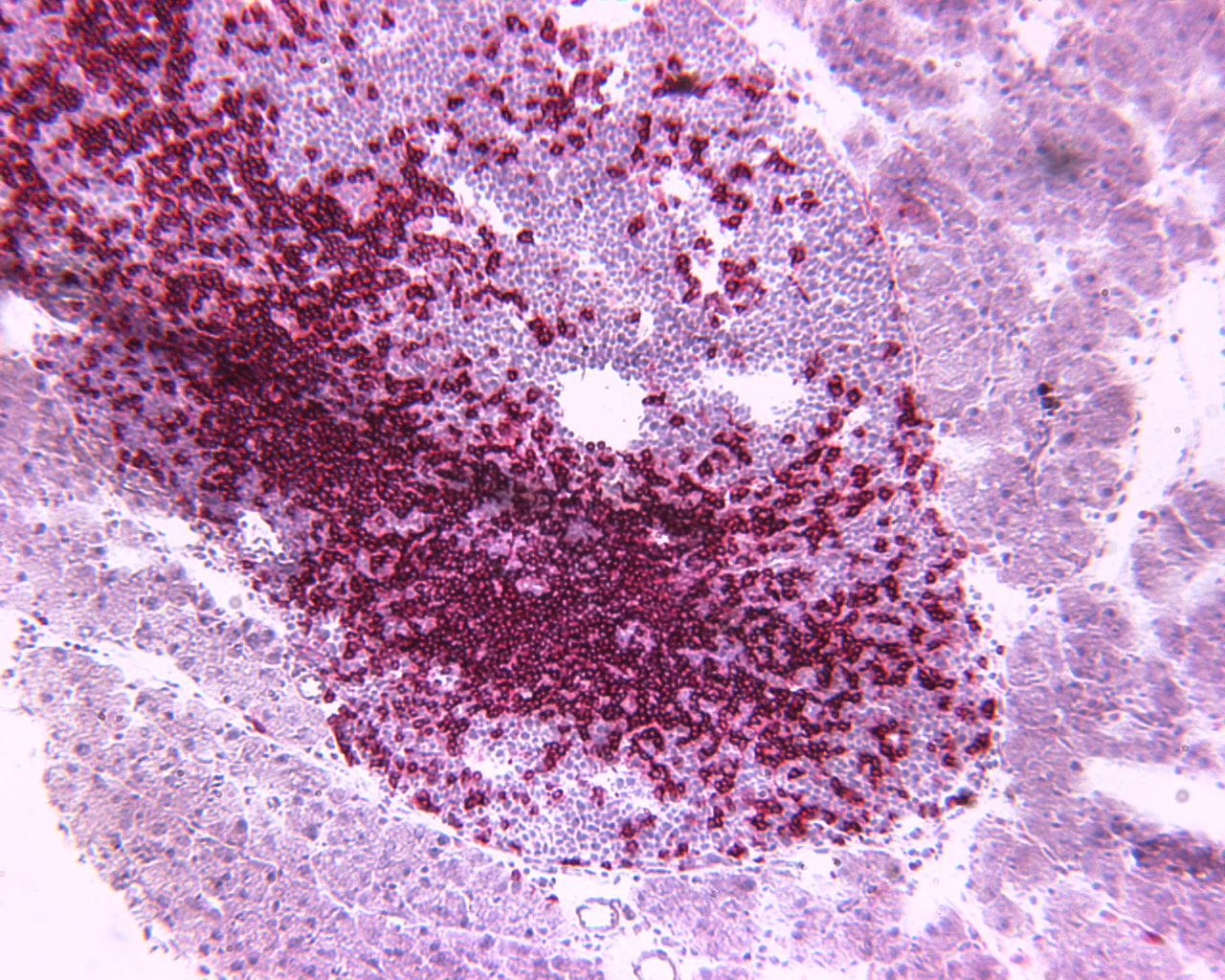 T cells (in red) entering a pancreatic islet
T cells (in red) entering a pancreatic islet
New Technologies and Infrastructure:
Infrastructure for running clinical trials: Our program has expertise in all aspects of clinical trials, including approval from Health Canada, trial set-up, patient accrual and trial follow up. Our team includes dedicated trial monitors who are focused on quality assurance. Together, we ensure that trials are conducted in accordance with institutional and regulatory guidelines and professional standards of practice.
Immune Monitoring: We have assembled a team of scientists with expertise in various state-of-the-art technologies for profiling the immune response of patients. This expertise comes together in the Immune Monitoring Team.
Cell Production Team: This team has expertise in producing cells for infusion into patients, such as T cells and dendritic cells. They also have expertise in the regulatory issues related to producing clinical-grade cells. In addition to producing clinical-grade cells, this team also provides cells for various research projects aimed at developing novel immune therapies.
New tool for detecting tumor-fighting T cells: Our group has developed a superior technology for detecting tumor-fighting T cells. The next steps will be to test this technology on patient samples, and share this technology with the world.
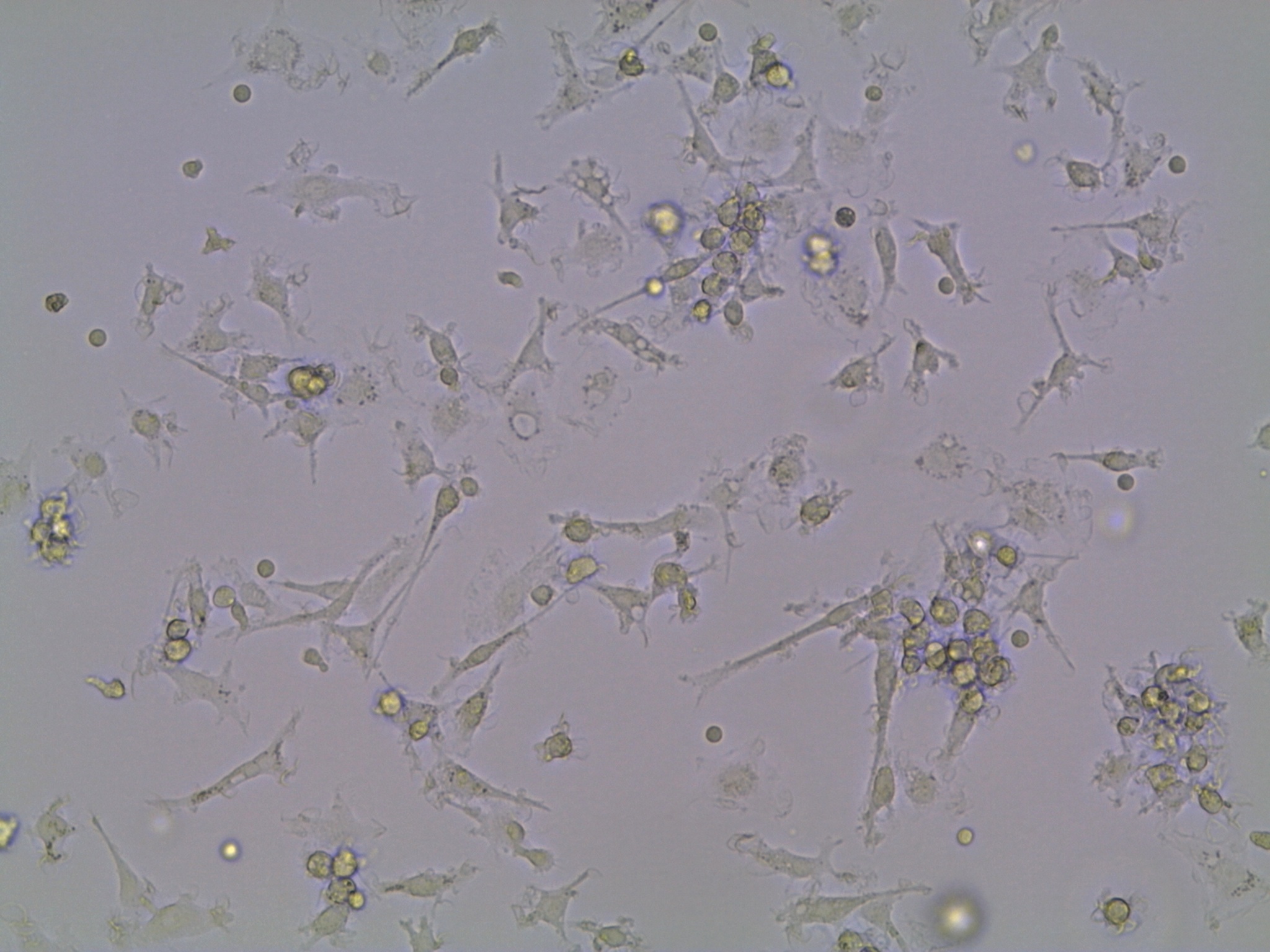 Dendritic cells growing in the lab.
Dendritic cells growing in the lab.
Tumor Immunotherapy Program (TIP) Database: We are developing a comprehensive database to capture clinical data from patients who have undergone immune therapy at the Princess Margaret. This bank of “big data” will include information about treatment regimens, side effects, clinical response, and patient survival. This comprehensive repository will be an invaluable tool for medical research. It will provide an important springboard to further cancer drug discovery and pave the way for a more personalized approach to cancer treatment.

